Description
The Alzheimer amyloid precursor protein (APP) is a transmembrane protein whose abnormal processing is associated with the pathogenesis of Alzheimer’s disease. APP695 lacking the protease inhibitor domain is the predominant form in neuronal tissues. APP695 is cleaved by caspases into the 664-residue amino (N)-terminal fragment that lacks the carboxyl C-terminal 31-residues (APPΔC31) and the 31-residues C-terminal fragment (APP-C31). Both fragments might be potent inducers of neuronal apoptosis. An antibody (named ACT1) against the N-terminus of caspase 3-generated APP C-terminal 31 aa of human APP695 (APP-C31) was raised in rabbit.
Applications
Western blot (dilution: 1/3,000-1/1,000)
Immunocytochemistry (dilution: 1/1,000-1/500)
ELISA
These applications were confirmed in the laboratory of Prof. K, Yoshikawa of Osaka University. (ref. 3).
Specification
Immunogen: Synthetic peptide corresponding to the N-terminus of the caspase 3-generated
APP C-terminal 31 amino acids (aa 665-670 of human APP695)
Specificity: Reactive to human, mouse and rat. Specific to the N-terminal end of the caspase 3-generated APP-C31
Form: Antiserum with 0.05% sodium azide
Storage: Shipped at 4°C and stored at -20°C
Data Link:
UniProtKB/Swiss-Prot P05067 (A4_HUMAN)
References: This antibody was used in ref. 3.
Kang HG et al (1987) “The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor.” Nature 325: 33-736 PMID: 2881207
Selkoe DJ (1994) “Normal and abnormal biology of the beta-amyloid precursor protein.” Annu Rev Neurosci 17: 489-517 PMID: 8210185
Nishimura I et al (2002) “Cell death induced by a caspase-cleaved transmembrane fragment of the Alzheimer amyloid precursor protein.” Cell Death Differ 9: 199-208 PMID: 11840170
Nishimura I et al (2003) “Upregulation and antiapoptotic role of endogenous Alzheimer amyloid precursor protein in dorsal root ganglion neurons.” Exp Cell Res 286: 241-251 PMID: 12749853
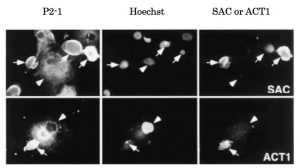
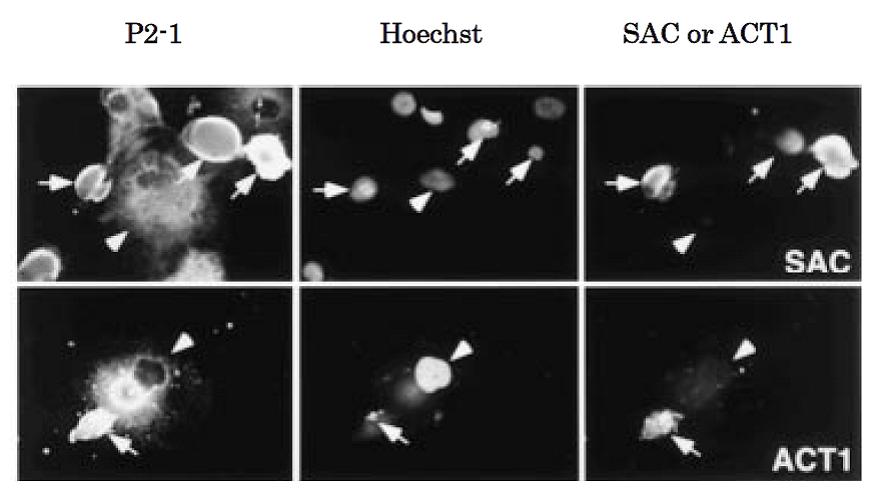
Fig.1 Immunocytochemistry for APPΔC31 and APP-C31: Generation of the caspase-cleaved fragments in NT2 neurons (neurally differentiated human NT2 embryonic carcinoma cells) overexpressing wild type APP (ref. 3). NT2 neurons were fixed 72 h after infection with adenovirus vector expressing wild-type APP and stained for the N-terminus of APP (P2-1, mouse monoclonal antibody), chromosomal DNA (Hoechst), the C-terminus of APPΔC31 (SAC) or the N-terminus of APP-C31 (ACT1). Most of wild-type APP-accumulating neurons with shrunken and fragmented nuclei contain SAC- and ACT1-immunoreactivities (arrows), but non-neuronal cells are hardly labeled with SAC and ACT1 (arrowheads).


Reviews
There are no reviews yet.