Description
Background: The glutamine- and asparagine-rich protein, Rnq1, is a putative yeast prion. Rnq1 protein with yet unknown function, can exists in either noninfectious soluble monomer form, [pin-], or the insoluble aggregated amyloid-like form called [PIN ]. The insoluble state is dominant and transmitted between cells through the cytoplasm (1). Rnq1 protein is necessary for the de novo induction of another prion, [PSI ] (2). The molecular chaperone Hsp104 is necessary for the aggregate formation of polyglutamine and for the maintenance of prion phenotype. The pre-existing aggregates are required for the chaperon-dependent establishment of the epigenetic trait in yeast prions (3).
Applications
1) Western blotting (300 fold dilution). Not tested for other applications.
Specifications
Product: Rabbit polyclonal antibody affinity purified with the synthetic peptide used as antigen
Immunogen: Synthetic peptide CSQQNNNGNQNRY corresponding to the C-terminus region of Rnq1
Form: Purified IgG in PBS, 1mg/ml BSA, 0.09% sodium azide, 50% glycerol
Reactivity: S. cerevisiae Rnq1, not tested with other species
Storage: -20°C (long-term storage at -70°C)
Data Link SGD RNQ1/YCL028W
References
This antibody is used in ref.3.
- Sondheimer N & Lindquist S “Rnq1: an epigenetic modifier of protein function in yeast” Mol Cell 5: 163-172 (2000) PMID: 10678178
- Derkatch IL et al “Effects of Q/N-rich, polyQ, and non-polyQ amyloids on the de novo formation of the [PSI ] prion in yeast and aggregation of Sup35 in vitro” Proc Natl Acad Sci USA 101:12934-12939 (2004) PMID: 15326312
- KimuraY et al “The role of pre-existing aggregates in Hsp104- dependent polyglutamine aggregate formation and epigenetic change of yeast prions” Genes to Cells 9: 685-696 (2004)
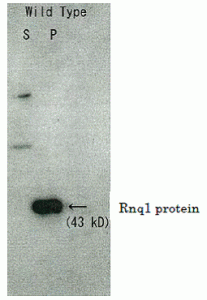
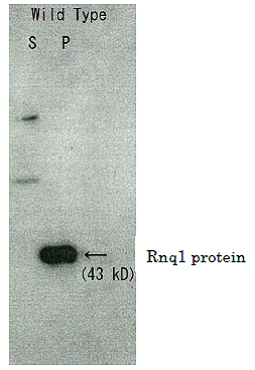
Fig.1 Detection of Rnq1 protein in S. cerevisiae by Western blotting with this antibody.
Cells were harvested after 24 h of galactose induction. Extracts were centrifuged and soluble (S) and pelleted (P) fractions were assayed by Western blotting using this antibody. Rnq1 protein was detected in pelleted fraction (ref. 3).


Reviews
There are no reviews yet.