Description
Description
Nucleoporin 98 (Nup98) is a component of nuclear pore complex (NPC), which is a large protein assembly embedded in the nuclear envelope and highly conserved in eukaryotes. It is localized on both nuclear and cytoplasmic side of NPC. This protein contains glycine-leucine-phenylalanine-glycine (GLFG) amino acid repeats and plays a critical role in nuclear trafficking. Nup98 also plays a specific role in the RNA export. In addition, Nup98 plays roles in several important biological events such as gene expression, mitotic checkpoint, and pathogenesis.
Nup98 gene is fused to a variety of partner genes in human myeloid and T-cell malignancies via chromosomal translocation.
In ciliates, a unicellular organism having two functionally distinct nuclei, GLFG-Nup98 is present in one of the nuclei and a distinct Nup98 ortholog is present in the other nucleus, and these different Nup98s participate in a nucleus-selective transport mechanism.
Clone 13C2
Applications
See Table 1 for summary. For details read Ref.1
1) Western blot – Human, S. cerevisiae, S. pombe, Tetrahymena. Not tested in other species.
2) Immunofluorescence staining and Immunocytochemistry for S. cerevisiae, S. pombe and Tetrahymena. Not suitable for IF in human
.
Specifications
Immunogen: Synthetic peptides containing conserved N-terminal sequence, GLFG, of Nup98 protein of Tetrahymena thermophila.
Peptide 1; 1-MFGNTGGGGLFGNTQTQQTGGGLFGQPQQ-29
Peptide 2; 646-SNPTQGGGLFGAANPGLGG-664
Epitope determined: FGxxN (Ref.1)
Reactivity: Human, S. cerevisiae, S. pombe and Tetrahymena. Not tested in other species
Isotype: Mouse IgG1 (κ)
Form: Purified IgG, 1 mg/ml in PBS(-), 50% glycerol, filter-sterilized. Azide- and carrier- free
Storage: Shipped at 4°C or -20°C, and upon arrival, spin-down and store at -20°C.
Clone 21A10
Applications
See Table 1 for summary. For details read Ref.1
1) Western blot for S. cerevisiae and S. pombe. Not suitable for human. Clone 13C2 is recommended for WB in human, Tetrahymena and yeasts.
2) Immunofluorescence staining and Immunocytochemistry for human, Tetrahymena, S. cerevisiae, S. pombe.
Specifications
Immunogen: Synthetic peptides containing conserved N-terminal sequence, GLFG, of Nup98 protein of Tetrahymena thermophila.
Peptide 1; 1-MFGNTGGGGLFGNTQTQQTGGGLFGQPQQ-29
Peptide 2; 646-SNPTQGGGLFGAANPGLGG-664
Epitope determined: GLF (Ref.1)
Reactivity: Human, S. cerevisiae, S. pombe and Tetrahymena. Not tested in other species
Isotype: Mouse IgG1 (κ)
Form: Purified IgG, 1 mg/ml in PBS(-), 50% glycerol, filter-sterilized. Azide- and carrier- free
Storage: Shipped at 4°C or -20°C, and upon arrival, spin-down and store at -20°C.
Data Link
UniProtKB P52948 NUP98_HUMAN
PomBase SPAC1486.05 Nup189, S. pombe
Reference
This product has been described and used in Ref. 1.
- Iwamoto M. et al. (2013) Monoclonal antibodies recognize gly-leu-phe-gly repeat of nucleoporin nup98 of tetrahymena, yeasts, and humans. Monoclon Antib Immunodiagn Immunother. 32: 81-90 PubMed ID: 23607342 WB, IF
- Iwamoto M. et al. (2010) Nucleoporin Nup98: a gatekeeper in the eukaryotic kingdoms. Genes Cells. 15: 661-9. PubMed ID: 20545767 Free article. Review
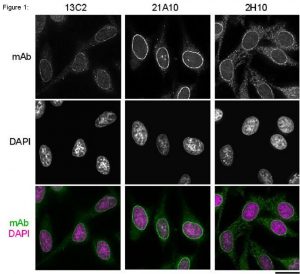
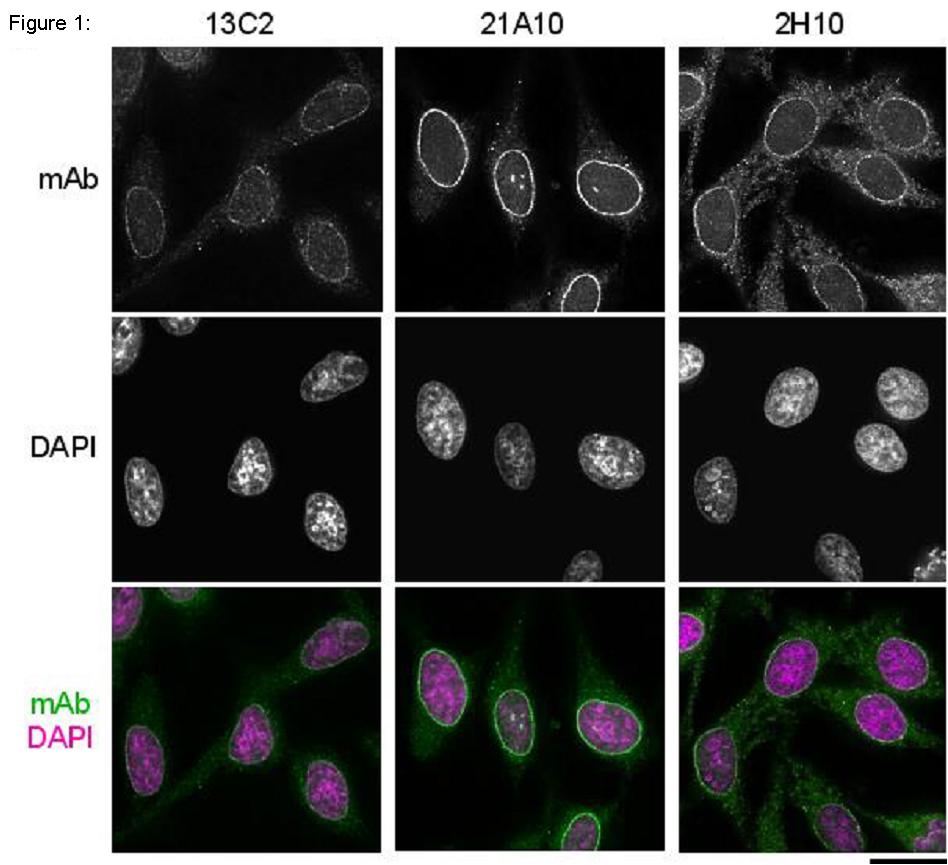
Fig.1 Immunofluorescene staining of Nup98 in HeLa cells using 13C2, 21A10, or 2H10 monoclonal antibodies. Black-and-white images were obtained with mAb and DAPI. Color images represent merged images of mAb (green) with DAPI (magenta).
HeLa cells were cultured in a glass-bottom dish (MatTek, Ashland, MA) for 2 days before fixation. The cells were fixed with cold methanol (-30°C) for 30 min. After washing with PBS, all fixed samples were blocked with 1% BSA for 2 hr at room temperature. mAbs 13C2 and 21A10 were diluted to 0.5 µg/ml in PBS. Anti-Nup98 rat mAb 2H10 was also diluted to 0.5 µg/ml in PBS. Blocked samples were treated with these primary antibody solutions overnight at 4°C. The secondary antibodies were 4 μg/ml of Alexa488-labelled anti-mouse IgG for 13C2 and 21A10 or anti-rat IgG for 2H10. DNA was stained with 4′, 6-diamidino-2-phenylindole (DAPI). Samples were washed three times with PBS between treatments. Fluorescence images were obtained with an Olympus fluorescence microscope IX-70 controlled by DeltaVision microscope system (Applied Precision, Issaquah, WA).
Both 13C2 and 21A10 mAbs stained the nuclear periphery of the human cells in a punctate pattern similar to that of 2H10 mAb. The signal at the nuclear periphery with 21A10 mAb was much higher and the background lower than that of 2H10 and 13C2 antibodies.
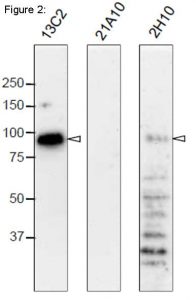
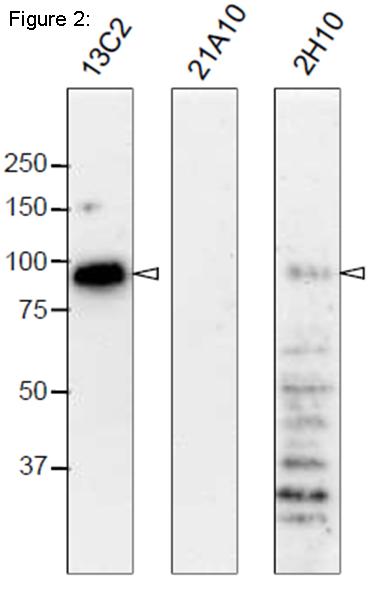
Fig. 2. Western blot analysis of Nup98 in HeLa cells with anti-Nup98 antibodies.
mAbs used are indicated on the tops of the lanes. Signals were detected using the same staining conditions, except that the exposure time of the middle lane (labeled 21A10 long exposure) was about 10 times longer than the exposure times of the other lanes. Open arrowheads represent the positions of Nup98. mAB 13C2 is the best for WB among them.
After SDS-PAGE, proteins were transferred to polyvinylidene difluoride (PVDF) membranes at 0.8 mA/cm2 for 2 hr using a semi-dry blotting apparatus. Membranes were blocked with 5% skim milk in PBS containing 0.1% Tween20 (PBS-T) for 3 hr at room temperature. The membranes were then incubated with mAbs diluted to 0.4 µg/ml in PBS containing 1% skim milk overnight at 4°C. Membranes were then washed three times for 10 min with PBS-T followed by incubation with 0.4 µg/ml horseradish peroxidase (HRP)-labeled anti-mouse IgG or anti-rat IgG for 13C2 and 21A10 or 2H10, respectively, for 2 hr at room temperature. The membranes were then incubated with ImmunoStar reagent (Wako) for 1 min, and chemiluminescence signals were detected by an image analyzer.
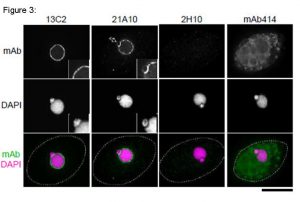
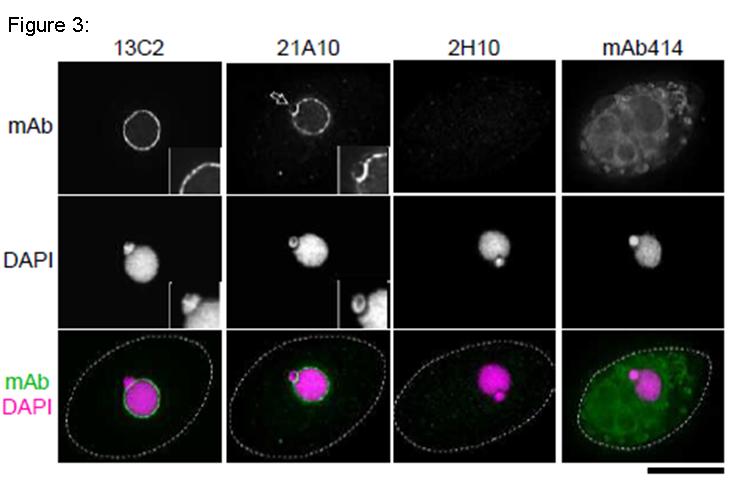
Fig. 3. Immunofluorescene staining of Nup98 in Tetrahymena themophila cells using 13C2, 21A10, 2H10 or 414 monoclonal antibodies. Black-and-white images were obtained with mAb and DAPI. Color images represent merged images of mAb (green) with DAPI (magenta). Dotted lines represent the outlines of cells. The open arrow indicates the micronucleus. Insets are magnified images showing the position of the micronucleus. Bar is 20 µm.
The cells were fixed with cold methanol (-30°C) for 30 min. After washing with PBS, all fixed samples were blocked with 1% BSA for 2 hr at room temperature. MAbs were diluted to 0.5 µg/ml in PBS. Blocked samples were treated with these primary antibody solutions overnight at 4°C. The secondary antibodies were 4 μg/ml of Alexa488-labelled anti-mouse IgG for 13C2, 21A10 and 414 or anti-rat IgG for 2H10. DNA was stained with 4′, 6-diamidino-2-phenylindole (DAPI). Samples were washed three times with PBS between treatments.
Both 13C2 and 21A10 mAbs stained the macronuclear periphery of T. thermophila. 13C2 mAb was highly specific to the macronucleus (see 13C2 “mAb” inset). In contrast, in addition to clear macronuclear staining, 21A10 mAb also stained the micronuclear periphery (see 21A10 “mAb” inset). This indicates that 21A10 mAb recognizes Nups localizing to the micronucleus such as Nup308 in addition to MacNup98A. Neither mABs 2H10 nor 414 could stain nuclear periphery of Tetrahymena.
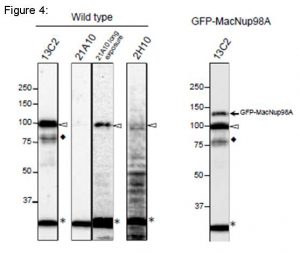
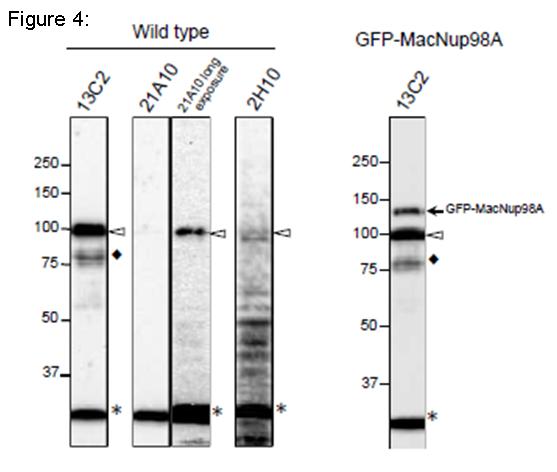
Fig. 4. Detection of MacNup98A of Tetrahymena by Western blot with monoclonal antibodies 13C2 and 21A10. WB analysis of T. thermophila specimen from wild type (left 4 lanes) or GFP-MacNup98A expressing cells (right most lane). mAbs used are indicated on the tops of the lanes. Signals were detected using the same staining conditions, except that the exposure time of the middle lane (labeled 21A10 long exposure) was about 10 times longer than the exposure times of the other lanes. Open arrowheads and the closed arrow represent the positions of MacNup98A and GFP-MacNup98A, respectively. Diamonds and asterisks represent uncharacterized proteins.
Both 13C2 and 21A10 mAbs detected a protein with an apparent molecular weight (MW) of 98 kDa. This value approximates the MW of MacNup98A (112 kDa) deduced from the DNA sequence. Ectopically expressing GFP-MacNup98A in addition to endogenous MacNup98A (see right most lane, labeled GFP-MacNup98A) were detected by 13C2, with MWs of 98 and 125 kDa in the samples (arrows in “GFP-MacNup98A”). The 125-kDa band corresponds to GFP-MacNup98A, and is approximately 28 kDa larger than the endogenous protein detected by 13C2. As the MW of GFP is 28 kDa, suggesting that the 98-kDa protein band is endogenous MacNup98A. 2H10 mAb, also detected a protein with the same MW as endogenous Nup98A, but the band was very faint. For detection of MacNup98A of Tetrahymena with Western blotting, 13C2 is the best among the three antibodies.
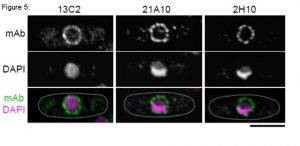
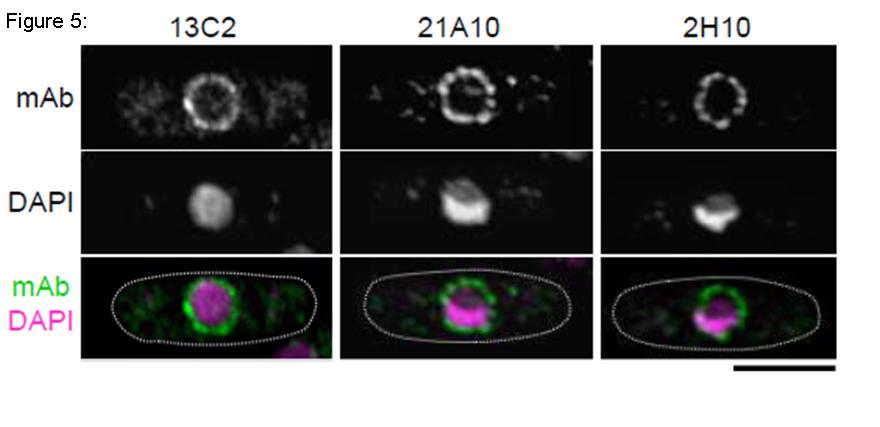
Fig. 5. Immunofluorescene staining of Nup98 in S. pombe cells with 13C2, 21A10, or 2H10 monoclonal antibodies. Black-and-white images were obtained with mAb and DAPI. Color images represent merged images of mAb (green) with DAPI (magenta). Dotted lines represent the outlines of cells. Bar is 5 µm. S. pombe cells were fixed with 4% formaldehyde for 10 min, treated with 0.6 mg/ml Zymolyase 100T at 36°C for 70 min, and permeabilized with 1% Triton X-100 for 1 min. 1/10 dilutions of the supernatants of hybridoma cultures of 13C2 or 21A10 or 10 µg/ml IgG solution of 2H10 were used. Both 13C2 and 21A10 mAbs stained the nuclear periphery of S. pombe in a punctate pattern similar to that of the 2H10 mAb
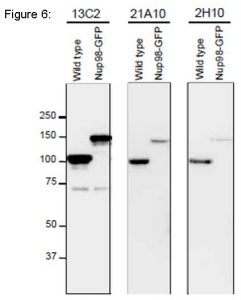
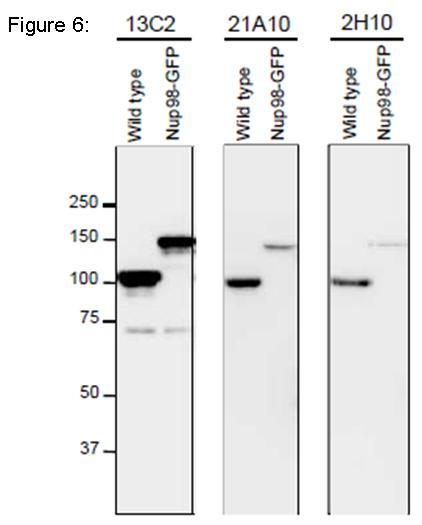
Fig. 6. Detection of Nup98 in S. pombe cell extracts by Western blotting with monoclonal antibodies 13C2, 21A10 and 2H10. 1/10 dilutions of the supernatants of hybridoma culture medium of 13C2 or 21A10 or 1 µg/ml IgG solution of 2H10 were used. Left and right lanes represent specimens from a wild type strain and an S. pombe strain in which Nup98 was chromosomally replaced with Nup98-GFP, respectively. S. pombe cells harvested were heated in distilled water at 95°C for 5 min, suspended in 100 mM phosphate buffer (pH 6.8), 4 M urea, 2.5% SDS, 0.05 mM EDTA, and disrupted by glass beads using a Multi Beads Shocker (Yasui Kikai, Osaka,). The sample was clarified by centrifugation, and the supernatant was used as the whole cell extract. WB was performed as described above. 13C2 antibody among the three gave best result for WB of Nup98 in S. pombe.
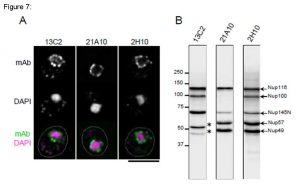
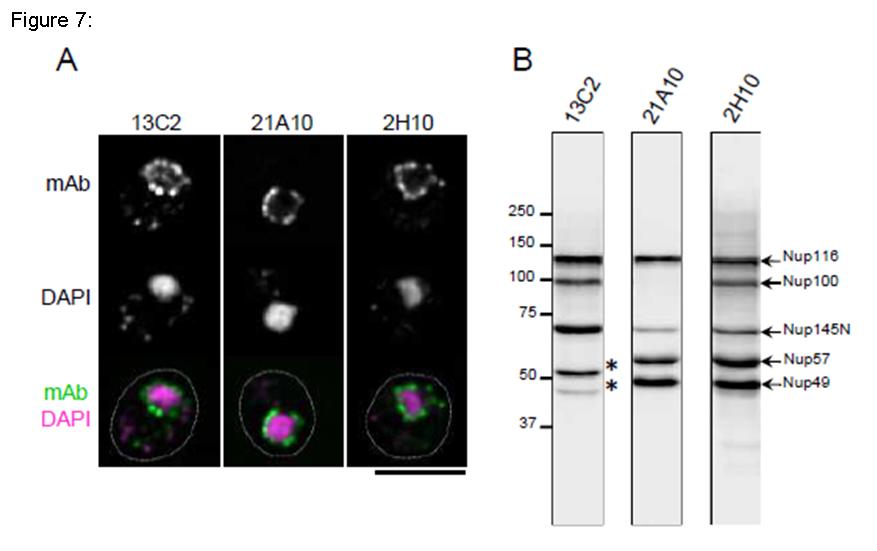
Fig. 7. Monoclonal antibodies 13C2 and 21A10 crossreact with multiple nucleoporins of S. cerevisiae.
(A) IF staining of S. cereviciae cells using 13C2, 21A10, or 2H10 mAbs: 1/10 dilutions of the supernatants of hybridoma cultures of 13C2 or 21A10 or 10 µg/ml IgG solution of 2H10 were used. Black-and-white images represent fluorescence images obtained with mAb (top) and DAPI (middle). Color images represent merged images of mAb (green) with DAPI (magenta). Dotted lines represent the outlines of cells. Bar is 5 µm. Methods were as described for S. pombe.
(B) WB analysis of S. cereviciae cell extract using 13C2 , 21A10, or 2H10 mAbs: 1/10 dilutions of the supernatants of hybridoma culture medium of 13C2 or 21A10 or 2 µg/ml IgG solution of 2H10 were used. Arrows represent the positions of the indicated nucleoporins. Asterisks represent uncharacterized proteins.
Methods were as described for S. pombe.
TABLE 1. Summary of the Suitability of mAbs for Immunological Applications
| T. thermophila | HeLa cell | S. cerevisiae | S. pombe | – | ||||||
| mAb | Isotype | IF | WB | IF | WB | IF | WB | IF | WB | epitope |
| 13C2 | Mouse IgG1 | +++ | +++ | + | +++ | +++ | +++ | +++ | +++ | FGxxN |
| 21A10 | Mouse IgG1 | +++ | + | +++ | – | +++ | ++ | +++ | ++ | GLF |
| 2H10 | Rat IgG2c | – | +/- | +++ | + | +++ | +++ | +++ | ++ | Unidentified |


Reviews
There are no reviews yet.